[ad_1]

Discover
In a hospital in Wuhan, China, a 41-year-old man struggles to breathe. He got here in on December 26, 2019, with a fever and flu-like signs, however docs can’t work out what’s ailing him. A number of different individuals at his office, an indoor seafood market, are additionally sick.
His docs run checks for influenza and different infections, however the outcomes come again unfavorable. Subsequent, they gather a pattern from his lungs by flooding his airway with a sterile saline resolution, then suctioning out the fluid.
The docs don’t understand it but, however in that pattern is a then-unknown virus. It invaded his lung cells, took over their equipment, and instructed them to churn out a military of latest viruses. In simply 10 hours, every contaminated cell creates sufficient new viruses to contaminate a thousand extra cells.
The code for these directions—and thus the important thing to defeating the virus—lies in its genome. This details about what’s ultimately referred to as SARS-CoV-2, the novel coronavirus that causes COVID-19, sits within the lung wash pattern in a freezer, ready to be “learn.”
ADVERTISEMENT
Log in
or
Be part of now
.
The Important Genome
For many of historical past, people didn’t perceive what made life tick. However over the previous 150 years, scientists have found that traits—traits as trivial as eye colour and as consequential as illness susceptibility—move from mother and father to their offspring. The important thing to this course of, heredity, is discovered within the genome inside every cell.
Genomes are important to all life types—even nonliving brokers like viruses. They embrace directions for constructing cells or viral particles and for what these cells or viruses ought to do. Genomes even comprise blueprints for passing these directions from mother and father to offspring.
Because the discovery of heredity, scientists have recognized the molecules that comprise genomes, deciphered their code, and discovered to “learn” and “write” the data. Now, scientists can learn the sequence of a genome in a day or two, on the push of a button. The machines that do that, now commonplace in analysis labs, have revolutionized how scientists detect, analyze, deal with, and forestall viral outbreaks.
ADVERTISEMENT
Log in
or
Be part of now
.
From the completion of the Human Genome Venture in 2003 to smaller however nonetheless important discoveries, sequencing has benefited from many years of philanthropic and governmental investments in fundamental science analysis.
“We’ve actually sequenced tens of hundreds of viruses,” says Craig Venter, PhD, the founder and CEO of the J. Craig Venter Institute and a frontrunner in genetics. In contrast with the early days of genome sequencing, he says, “the method immediately may be very routine, very quick, and really cheap.”
However immediately’s sequencing expertise wouldn’t have been potential if it weren’t for generations of scientists who unraveled the genome and discovered the way it works. From the completion of the Human Genome Venture in 2003 to smaller however nonetheless important discoveries, sequencing has benefited from many years of philanthropic and governmental investments in fundamental science analysis.
“I’m not a geneticist, however the applied sciences that I developed have answered big questions that individuals had for years about genetics,” says biomedical engineer David Walt, PhD. A Howard Hughes Medical Institute Investigator at Harvard’s Wyss Institute for Biologically Impressed Engineering who has cofounded a number of biotech corporations. “I couldn’t have recognized what questions the ten,000—maybe 100,000—papers which have used my applied sciences would attempt to reply,” provides Walt, “however I’m glad I used to be capable of allow these research.”
ADVERTISEMENT
Log in
or
Be part of now
.
The Heredity Molecule
The earliest clues to the mysteries of inheritance emerged within the 1860s, when Austrian monk Gregor Mendel experimented with pea crops. By mating crops with totally different traits, Mendel noticed which traits—colour, measurement, or form, for instance—have been handed on and the way usually. However he couldn’t establish how the traits have been transferred.

ADVERTISEMENT
Log in
or
Be part of now
.
As Mendel was mating peas, Swiss doctor and chemist Friedrich Miescher, MD, was conducting analysis that ultimately led to the identification of the substance containing hereditary info. Whereas inspecting white blood cells from pus-filled bandages, Miescher remoted a novel part of the cells that he referred to as “nuclein.” Over 70 years later, scientists realized that Miescher’s nuclein is what confers heredity.
In 1944, Oswald Avery, MD, a researcher on the Rockefeller Institute for Medical Analysis in New York made a breakthrough whereas learning pneumococci micro organism. There are two types of this bacterium, R and S; the S type is extra lethal, the R much less so. However the trait can move between the 2 forms of micro organism. By learning the elements that turned R sorts into S sorts, he confirmed that these molecules had the chemical and bodily properties of nuclein.
However one other decade handed earlier than researchers totally appreciated Avery’s discovery, thanks largely to work on the College of Cambridge by American biologist James Watson, PhD, and English physicist Francis Crick, PhD. In 1953, utilizing X-ray crystallography photographs by chemist Rosalind Franklin, PhD, Watson and Crick assembled cardboard cutouts of deoxyribonucleic acid (DNA) to find out how its elements match collectively. Their work led to the publication of the double helix mannequin. This mannequin was instrumental in transferring analysis ahead which confirmed {that a} being’s genome, encoding the construction and performance of each cell, is inscribed in DNA.
We now know {that a} molecule much like DNA, ribonucleic acid (RNA), serves as an middleman inside cells. It’s usually a duplicate of DNA that will get translated to proteins by the cell’s ribosomes. Multicellular organisms like people and crops, in addition to microbes like micro organism and even some viruses, have genomes fabricated from DNA. However many viruses, together with SARS-CoV-2, retailer their genetic code as RNA. RNA viruses bypass the DNA step in protein manufacturing, to allow them to reproduce sooner and infect extra cells. Additionally they evolve sooner than DNA viruses, in order that they’re extra more likely to mutate and achieve new traits.
ADVERTISEMENT
Log in
or
Be part of now
.
Isolating RNA In China
In Wuhan, increasingly circumstances of the mysterious sickness are reported. On January 3, the 41-year-old affected person’s lung wash pattern arrives at Fudan College’s Shanghai Public Well being Scientific Middle. Researchers within the lab of virologist Yong-Zhen Zhang, PhD, put together the pattern.
Utilizing a preformulated package of chemical compounds, scientists isolate the pattern’s RNA and assess its high quality and amount. Then, utilizing one other package, they take away the host cell’s ribosomal RNA—the RNA part of ribosomes—and break the remaining viral RNA into quick sequences. A code is added to the ends of the RNA items, to bind them to the machine, and a bar code is added to every piece for monitoring.
ADVERTISEMENT
Log in
or
Be part of now
.
The genetic materials from the lung wash pattern is able to be sequenced.
Sanger Solves Sequencing
Within the late Fifties, a couple of years after Watson and Crick revealed their mannequin of DNA, American biochemist Arthur Kornberg, MD, introduced scientists one step nearer to creating a course of for sequencing a genome. At Washington College in St. Louis, he was the primary to isolate crucial enzyme within the genetic copying course of, DNA polymerase—which turns one DNA strand into two when a cell divides—work that gained him a share of the 1959 Nobel Prize.
ADVERTISEMENT
Log in
or
Be part of now
.
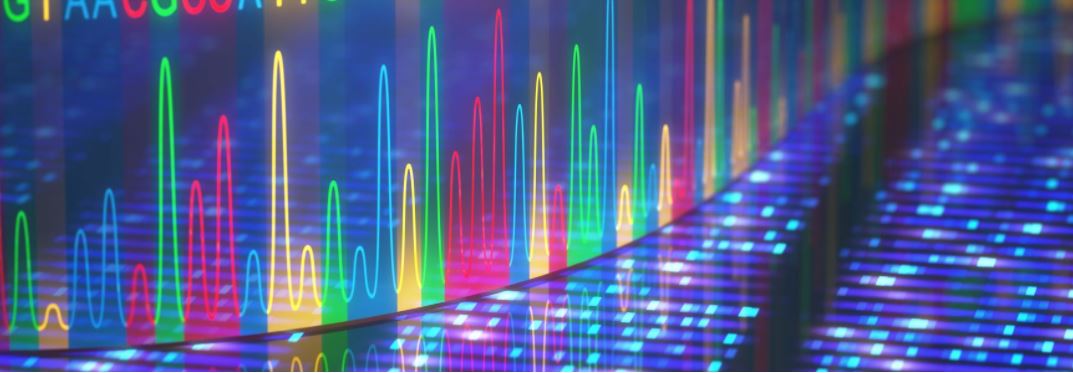
However two extra many years handed earlier than British chemist Frederick Sanger, PhD, a two-time Nobel laureate, harnessed the cell’s copying equipment to quickly reveal the sequence of letters in a strand of DNA. DNA sequences are made up of 4 bases which can be very comparable in measurement, form, and make-up however that differ barely of their chemistry: adenine (A), cytosine (C), guanine (G), and thymine (T).
To “learn” the letters utilizing Sanger’s sequencing methodology, researchers ran 4 experiments facet by facet—one for every letter. In every experiment, the researcher put all of the mobile elements which can be wanted to repeat DNA, together with three regular DNA bases—say, A, T, and C–and a dideoxynucleotide fourth base, on this case G. Dideoxynucleotide bases lack the hydroxyl group required for extension of DNA chains. When integrated within the strand, the cell’s equipment wouldn’t have the ability to add extra bases to the rising DNA strand, stalling the copying course of. To visualise the DNA, every experiment included a radioactive or fluorescent label that may get integrated within the copied strand and might be visualized utilizing X-ray or fluorescent imaging instruments.
Every of the 4 experiments used a unique dideoxynucleotide base. The researchers then added the DNA they wished to sequence to all 4 experiments, and the mobile equipment would copy it. When the dideoxynucleotide base is integrated, the DNA strand would cease from rising.
Every experiment created many DNA items of various lengths, however all ended with the precise dideoxynucleotide base. The items of DNA from every of the 4 experiments have been then separated by size, facet by facet, and visualized utilizing the label to see what order they have been in. The order of the DNA bands informed scientists the order of the bases.
ADVERTISEMENT
Log in
or
Be part of now
.
For instance, if the order of a strand of DNA was ATCG, the shortest band would present up within the A experiment; the second-shortest within the T experiment; the three-base strand within the C experiment; and the longest strand within the G experiment. “This was a very handbook approach,” Venter says. “It was tedious, gradual, and costly.”
Making Use of Knowledge
Sanger’s methodology, although tedious, was extra fast and correct than the earlier methodology, which was used to efficiently decode the complete viral genome of the virus phi X174 for the primary time in 1977. The 5,375 bases of its genome have been written by hand within the notebooks of 9 researchers. The subsequent step was to determine what to do with this painstakingly compiled knowledge.
ADVERTISEMENT
Log in
or
Be part of now
.
On the U.S.’s Los Alamos Nationwide Laboratory, nuclear physicist Walter Goad, PhD, was preserving monitor of genetic sequences being decoded in labs all over the world. His work, together with that of Margaret Dayhoff, PhD, a bodily chemist at Georgetown College Medical Middle, led to the event of a nationwide genetic sequence database—generally known as GenBank—on the Nationwide Institutes of Well being (NIH). This database allows researchers to identify similarities and variations amongst varied genes—to see how they alter over time and to pinpoint what variations change how the genes work.
Discovering these similarities and variations isn’t straightforward. Lengthy swaths of bases is perhaps added or deleted, making it troublesome to manually discover the place modifications have occurred. Lining up sequences takes critical laptop energy, particularly as sequences get longer and modifications better.
It was such a recreation changer in analyzing DNA that it was referred to as the Google of organic analysis, a bioinformatics killer app. Researchers worldwide nonetheless use its many iterations.
ADVERTISEMENT
Log in
or
Be part of now
.
Genetics researchers wanted particular software program to line up the extra advanced sequences—and so they have been aided by somewhat serendipity. In 1982, David Lipman, MD, a postdoc on the NIH, crossed paths with programmer Tim Havell, who talked about that the search instruments of the working system UNIX is perhaps helpful for cataloging organic sequences.
Working from this concept, Lipman and his collaborators created a pc algorithm to match up DNA sequences. Their work ultimately led to the discharge in 1990 of what’s generally known as the BLAST (fundamental native alignment search software) algorithm. It was such a recreation changer in analyzing DNA that it was referred to as the Google of organic analysis, a bioinformatics killer app. Researchers worldwide nonetheless use its many iterations.
Competitors Spurs Sequencing
ADVERTISEMENT
Log in
or
Be part of now
.
As computer systems improved, the so-called “shotgun” sequencing methodology, first urged in 1979, got here into favor. This concerned breaking apart the genome and sequencing every strand individually so complete genomes may very well be decoded extra rapidly. However lining up all the information from hundreds of thousands of quick strands isn’t one thing researchers may do by hand; they relied increasingly on algorithms to do the heavy lifting.
In the meantime, fundamental analysis from different disciplines—engineering, physics, astronomy, laptop science—laid the inspiration for so-called next-generation sequencing, which depends on the detection of fluorescent bases by imaging sensors.
Additionally they relied extra on automation. The primary computerized Sanger sequencing machines have been produced in 1987 by Utilized Biosystems. However as a substitute of Sanger’s radioactive bases, they used safer fluorescent bases. Lasers shot on the fluorescent bases made them give off gentle of sure colours, which computer systems may learn.
This expertise—mixed with improved strategies, higher laptop packages, and better processing energy—opened the genome floodgates, together with funding and competitors fueled by the launching of the Human Genome Venture in 1990.
ADVERTISEMENT
Log in
or
Be part of now
.
By the point the primary working draft of the human genome was revealed in 2001—in two papers, one from the NIH and one from Venter’s group—the time, effort, and value of sequencing had dropped dramatically. Some funding for the undertaking got here from Congress, however a lot got here from non-public philanthropy and trade. “The federal government isn’t good at funding new concepts,” says Venter. “They turned down our proposal to sequence the primary genome, being sure that our strategies wouldn’t work.”
In the meantime, fundamental analysis from different disciplines—engineering, physics, astronomy, laptop science—laid the inspiration for so-called next-generation sequencing, which depends on the detection of fluorescent bases by imaging sensors. In 1969, Bell Labs researchers sketched out the primary charge-coupled system, now generally known as a CCD sensor, altering digital imaging eternally. By the late Nineteen Eighties, superior CCDs developed for the Galileo mission to Jupiter may pick tiny pinpoints of sunshine from the sky.
As sensors received smaller and smaller, microengineering strategies expedited the miniaturization and automation of sequencing machines. They turned simpler, sooner, and cheaper to make and shortly have been obtainable to scientists in all places.
These new sequencing applied sciences proved extraordinarily beneficial in the course of the mid-2010s Ebola outbreaks. “Earlier than the Ebola outbreak, genomic epidemiology was extra retrospective than actual time,” says College of Arizona evolutionary biologist Michael Worobey, PhD. “This was the primary time you had individuals making an attempt to generate genomes of the virus on the similar time {that a} large, necessary outbreak was occurring.”
ADVERTISEMENT
Log in
or
Be part of now
.
Between 2014 and 2016, Ebola killed greater than 11,000 individuals in West Africa. Sequencing was important to the response on the bottom, serving to Harvard computational geneticist Pardis Sabeti, MD, perceive how the virus was spreading and the place it got here from.

“The genome is a dwelling historical past, all the time altering,” says Sabeti. It “permits us to trace and perceive when a virus emerged, the way it’s altering, the way it’s spreading,” she explains. “It’s a foundational, elementary, important piece.” When Ebola hit, her workforce despatched affected person samples again to Boston for sequencing—proving the outbreak was spreading individual to individual.
“That was the primary time actually large-scale genomics was used,” says College of Sydney geneticist Edward Holmes, PhD. “I feel we received one thing like 1,500 to 1,600 full genomes of the virus. That was a giant deal,” he provides, although it took weeks to show these genomes round.
ADVERTISEMENT
Log in
or
Be part of now
.
“In SARS-CoV-2,” Holmes continues, “we’ve now received greater than 400,000 genomes, and so they’re finished inside 24 hours.” Even so, the next-generation sequencing of these 400,000 genomes was hauntingly much like Sanger’s approach. However immediately’s sequencers analyze for much longer strands of genetic materials, a lot sooner, and far more cheaply, on the push of a button.
The evolution in sequencing has been exceptional, agrees geneticist George Church, PhD, of Harvard’s Wyss Institute. “It’s gone from one thing form of heroic and really artisanal,” he says, to one thing so routine that “it’s virtually like checking a field.”
At present, one tiny chip can perform billions of sequencing reactions without delay. “It’s 100 to a trillion instances cheaper,” Church explains. “It takes up the identical quantity of area . . . however is much more efficient.”
The genetic materials in a pattern is connected to a plastic chip that’s inserted into the machine. A number of rounds of automated reactions—the addition of enzymes, bases, and repeated washings—flip every sequence within the pattern into an island of RNA strands. These strands wave about on the chip like hundreds of thousands of tiny inflatable air dancers, able to be sequenced.
ADVERTISEMENT
Log in
or
Be part of now
.
If sequencing expertise ready us for the COVID-19 pandemic, that’s solely due to the many years of fundamental science analysis that preceded it.
The machine then cycles by means of reactions that develop an identical strand of RNA connected to every one on the chip. When a fluorescent base is added, it stops the RNA strand from rising and offers off a coloured sign. The machine takes a digital image of each addition, studying the colour as a letter base, then cycles by means of extra reactions. The system reads all of the islands of genetic materials concurrently, coloured base by coloured base.
After studying the strands letter by letter, the machine’s laptop traces them up in a full genome, then compares it utilizing BLAST algorithms to recognized viral genomes in databases like GenBank.
ADVERTISEMENT
Log in
or
Be part of now
.
The Wuhan Sequence
On January 5, the agent within the Wuhan affected person’s pattern is recognized and named. Its sequence is 90% an identical to SARS, which killed tons of of individuals in 2003. “It was fairly apparent,” Holmes says later, “that this virus was like the primary SARS coronavirus. That was an ‘Oh, s–t!’ second.”
The brand new virus’s similarity to SARS means it could actually seemingly unfold from human to human as simply as SARS, if no more so. It’s obvious a pandemic is brewing in Wuhan. Zhang instantly submits SARS-CoV-2’s genome to GenBank. Subsequent, after speaking with Zhang, Holmes publishes the sequence on January 10 on virological.org, so the scientific neighborhood can begin utilizing it to develop therapies, checks, and vaccines. “At that time, it was fairly apparent to us this was a human transmissible virus,” Holmes displays a number of months later. “Sadly, it took a number of weeks for that to be acknowledged.”
The 41-year-old Wuhan affected person’s immune system beats again the virus, and he survives. Others in Wuhan aren’t as fortunate. In only a week between the gathering of his lung wash pattern and its sequencing, over 600 extra individuals present signs of COVID-19.
ADVERTISEMENT
Log in
or
Be part of now
.
Investing In The Future
If sequencing expertise ready us for the COVID-19 pandemic, that’s solely due to the many years of fundamental science analysis that preceded it.
“Once I began, most critical scientists would select to do fundamental analysis, and I feel we’re reaping the harvest of that,” Church says. “However we’re additionally consuming our seed corn. If we don’t preserve doing fundamental analysis, we may get caught unprepared when there’s a brand new class of menace.”
ADVERTISEMENT
Log in
or
Be part of now
.
“Philanthropy is what provides researchers like myself, and analysis establishments just like the Venter Institute, an opportunity to do experiments and to develop new areas,” says Venter. “These experiments wouldn’t occur in any other case.”
The expertise or software that we’ll must battle the following pandemic, seemingly attributable to a now-unknown pathogen, will in all probability spring from experiments undertaken and not using a particular purpose. “The analysis areas that we might look again on and say ‘Wow, that was being disregarded, left behind’ are issues we are able to’t even consider now,” says Church. “So that you want scientists who’re actually paid to ‘play’—to only consider random issues.”
Which means funding in fundamental analysis is critical immediately, to put the groundwork for tomorrow’s next-generation considering. “Philanthropy is what provides researchers like myself, and analysis establishments just like the Venter Institute, an opportunity to do experiments and to develop new areas,” says Venter. “These experiments wouldn’t occur in any other case.”
Zhang’s SARS-CoV-2 sequence was simply the primary of tons of of hundreds added to GenBank. By the tip of the pandemic, scientists will seemingly have sequenced over one million SARS-CoV-2 genomes. There will probably be plenty of knowledge for researchers to “play” with.
ADVERTISEMENT
Log in
or
Be part of now
.
“Genome sequencing has been a transformative software,” says Walt, “one which we’ve exploited throughout this pandemic. When you look globally, we’re now capable of monitor the migration of the virus and the evolution of the virus with unprecedented element and determination.”
The fruits of this labor are already evident. For instance, we all know {that a} pressure initially detected within the U.Ok. spreads extra simply. Scientists have been capable of detect this pressure due to a fast, widespread sequencing program referred to as the COVID-19 Genomics U.Ok. Consortium. Funded by the British authorities and the Wellcome Sanger Institute, this system analyzes samples from throughout the nation to detect and monitor modifications within the virus’s genome. To this point it’s sequenced about 10% of all U.Ok. infections.
Genome sequencing cannot solely hint a pandemic because it evolves, but in addition bolster defenses in opposition to it. Researchers created the primary COVID-19 diagnostic check utilizing Zhang’s sequence simply two days after Holmes revealed it. “We went from first case to a diagnostic check in six weeks—I imply, it was simply extraordinary,” says Walt. “It’s simply an unimaginable accomplishment of science.”
ADVERTISEMENT
Log in
or
Be part of now
.
And utilizing Zhang’s sequence, Moderna was capable of synthesize a vaccine in a couple of days. In December 2020, it was granted Emergency Use Authorization by the U.S. Meals and Drug Administration. Inside a couple of months, only a yr after COVID-19 was declared a pandemic, hundreds of thousands of individuals had been vaccinated in opposition to it. “That’s completely, traditionally necessary,” Worobey says. “We’ve by no means had a vaccine so rapidly, and that comes instantly from the power to sequence the genome of the virus.”
[ad_2]
Supply hyperlink